Detailed Description
Potential functions for covalent dihedral interactions.
Dihedral potentials are usually expressed as functions of a dihedral torsion angle, 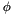 . Dihedral interactions may use instances of Torsion and TorsionForce to calculate the dihedral angle and its derivatives. The dihedral angle is defined in these classes as follows:
. Dihedral interactions may use instances of Torsion and TorsionForce to calculate the dihedral angle and its derivatives. The dihedral angle is defined in these classes as follows:
Consider 4 sequential atoms with position vectors 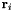 labelled sequentially by an index i = 0, 1, 2, 3. We define three bond vectors
labelled sequentially by an index i = 0, 1, 2, 3. We define three bond vectors
![\[ {\bf b}_i = {\bf r}_i - {\bf r}_{i-1} \]](form_36.png)
for i = 1, 2, 3. The dihedral angle is an angle between the plane spanned by bond vectors 1 and 2 and the plane spanned by bond vectors 2 and 3. Define two vectors
![\[ {\bf v}_{1} = {\bf b}_1 \times {\bf b}_2 \quad\quad {\bf v}_{2} = {\bf b}_2 \times {\bf b}_3 \]](form_37.png)
perpendicular to these planes, and corresponding unit vectors
![\[ {\bf u}_1 = {\bf v}_{1}/|{\bf v}_{1}| \quad\quad {\bf u}_2 = {\bf v}_{2}/|{\bf v}_{2}| \]](form_38.png)
The cosine of the dihedral angle is given by the dot product:
![\[ \cos(\phi) \equiv {\bf u}_1 \cdot {\bf u}_2 \]](form_39.png)
With this convention, a planar cis (arc) conformation yields phi = 0, and a planar trans (zig-zag) conformation yields phi = 180 degrees.
Classes | |
| class | Simp::CosineDihedral |
| A dihedral potential proportional to cos(phi). More... | |
| class | Simp::MultiHarmonicDihedral |
| A truncated Fourier series dihedral potential. More... | |
| struct | Simp::Torsion |
| Computes dihedral / torsion angle involving 3 bonds. More... | |
| struct | Simp::TorsionForce |
| Computes derivatives of dihedral angle with respect to bond vectors. More... | |
 1.8.11
1.8.11