Each of the dihedral interaction classes defines a potential that depends upon the dihedral angle involving two bonds that connect four atoms. Each such group of atoms is assigned an dihedral type index, and different values of the parameters in the dihedral potential function are used for different dihedral types.
All of the dihedral interaction classes implement a common interface, which is described here.
Geometry
Let the three atoms in an dihedral group be labelled 0, 1, 2, 3, with bonds connecting 0-1 and 1-2. Let 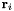 be the position vector for atom i, with
be the position vector for atom i, with 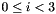 . We define three bond vectors
. We define three bond vectors
![\[ {\bf b}_{1} \equiv {\bf r}_{1} - {\bf r}_{0} \quad\quad\quad {\bf b}_{2} \equiv {\bf r}_{2} - {\bf r}_{1} \quad\quad\quad {\bf b}_{3} \equiv {\bf r}_{3} - {\bf r}_{2} \]](form_171.png)
The dihedral potentials are all function of the dihedral angle 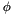 . This angle
. This angle 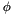 is the angle between the plane spanned by
is the angle between the plane spanned by 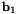 and
and 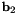 the plane spanned by
the plane spanned by 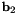 and
and 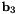 . It is defined so that a planar cis configuration corresponds to
. It is defined so that a planar cis configuration corresponds to 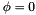 , and a trans zigzag configuration corresponds to
, and a trans zigzag configuration corresponds to 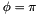 . A more explicit expression for
. A more explicit expression for 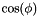 is given in Dihedral Interactions.
is given in Dihedral Interactions.
Dihedral Interaction Classes
- CosineDihedral - proportional to cosine of dihedral angle
- MultiHarmonicDihedral - Fourier expansion in dihedral angle
 1.8.11
1.8.11